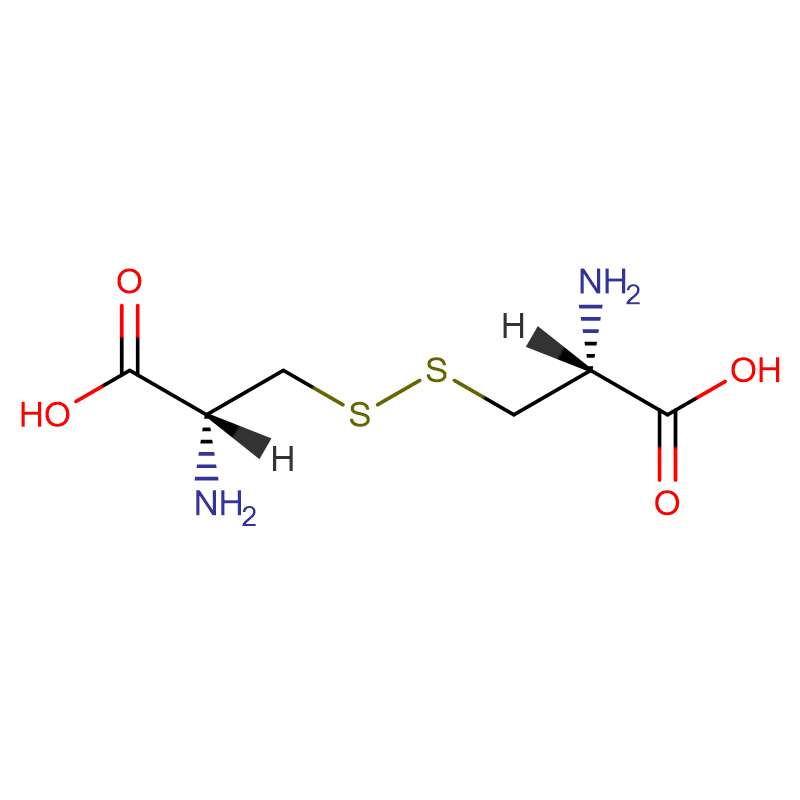
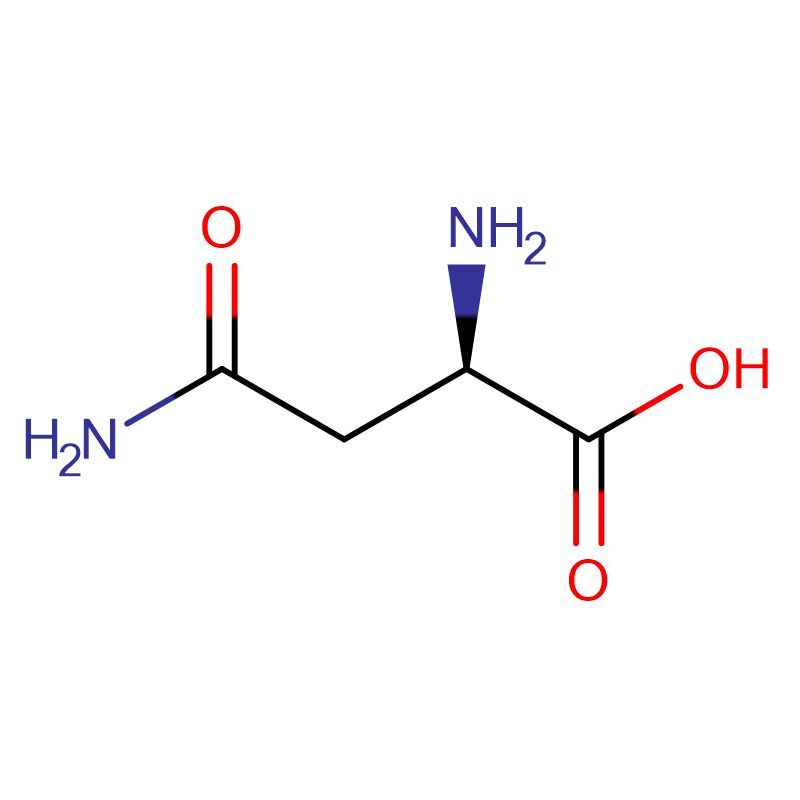
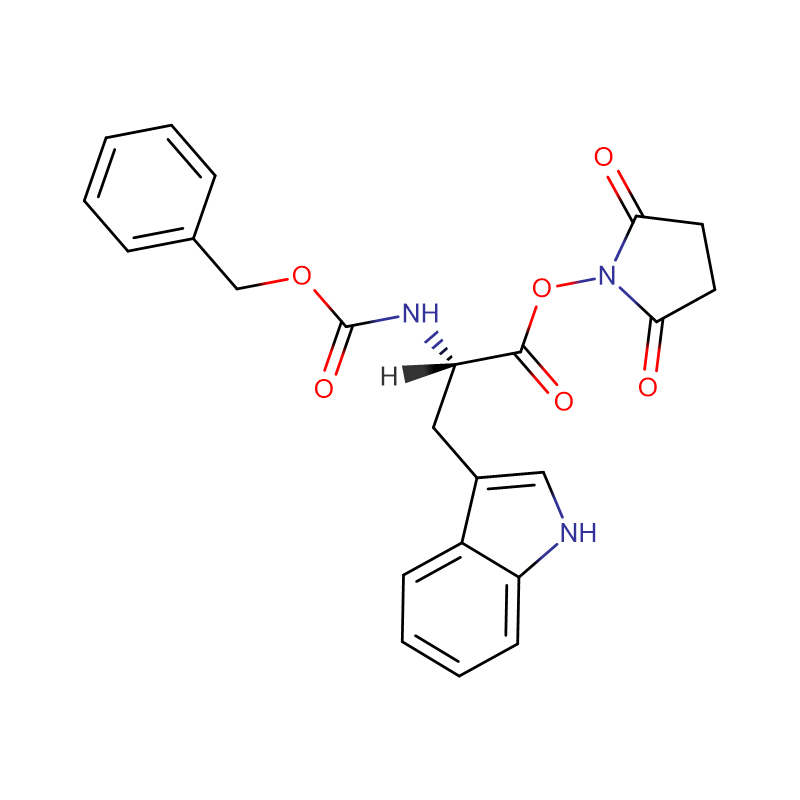
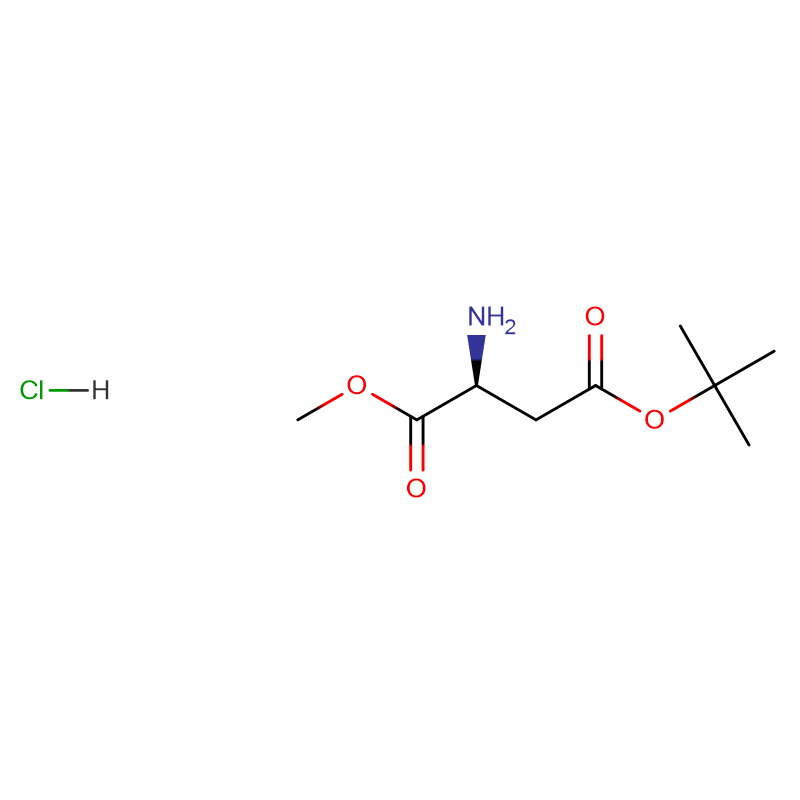
L-Cystin CAS: 56-89-3 99% crystals cad ama budada crystalline
| Lambarka buugaagta | XD90322 |
| Magaca Alaabta | L-Cystin |
| CAS | 56-89-3 |
| Qaanuunka molecular | C6H12N2O4S2 |
| Miisaanka Molecular | 240.30 |
| Faahfaahinta Kaydinta | Ambient |
| Xeerka Tarifka La Iswaafajiyay | 29309013 |
Tilmaamaha Alaabta
| Muuqashada | Kiristaalo cad ama budada crystalline |
| Qiimayn | 99% |
| Darajo | USP32 |
| Wareegid gaar ah | -215° ilaa -225° |
| Biraha culus | <0.0015% |
| AS | ugu badnaan 1.5ppm |
| SO4 | 0.040% ugu badnaan |
| Fe | <0.003% |
| Khasaare xagga qallajinta | 0.20% ugu badnaan |
| Hadhaaga dabka | 0.10% ugu badnaan |
| Cl | 0.10% ugu badnaan |
Qaab dhismeedka crystal-carboksypeptidase T (CpT) oo leh phenylalanine iyo arginine substrate analogs - benzylsuccinic acid iyo (2-guanidinoethylmercapto) succinic acid - waxaa lagu go'aamiyay habka beddelka molecular ee qaraarada 1.57 Å iyo 1.62 Å si loo caddeeyo astaanta hoose ee gaarka ah. ee enzyme.Haraaga muxaafidka ah ee Leu211 iyo Leu254 (sidoo kale ku jira labada carboxypeptidase A iyo carboxypeptidase B) ayaa la muujiyay inay yihiin go'aamiye qaab dhismeedka aqoonsiga substrates hydrophobic, halka Asp263 ay ahayd aqoonsiga substrates si togan loo dallacay.Isbeddellada go'aamiyeyaashan waxay wax ka beddelaan astaanta substrate-ka: kala duwanaanshaha CpT Leu211Gln wuxuu helayaa guryaha carboxypeptidase B, iyo kala duwanaanshaha CpT Asp263Asn carboxypeptidase A-doorasho la mid ah.Loop-ka Pro248-Asp258 ee la falgalaya Leu254 iyo Tyr255 waxa la tusay inay masuul ka yihiin aqoonsiga hadhaaga C-terminal substrate.Ku xidhida substrate-ka hoose ee S1' waxay keenaysaa isbeddelka isku xidhka-ku-tiirsanaanta ee wareeggan, iyo dhaqdhaqaaqa silsiladda dhinaca Leu254 waxay keenaysaa dib-u-habaynta qaabaynta hadhaaga Glu277 ee muhiimka u ah catalysis.Tani waa aragti cusub oo ku saabsan xulashada substrate-ka ee metallocarboxypeptidase taas oo muujinaysa muhiimada isdhexgalka ka dhexeeya S1' hoosaadka iyo xarunta katalytic.